
LEVAGEN®+ : A HIGH QUALITY, HIGHLY BIOAVAILABLE AND SCIENTIFICALLY PROVEN PEA
Palmitoylethanolamide (PEA) is an endogenous endocannabinoid receptor agonist. Present in small quantities in our diet (soybeans, peanuts and egg yolks), PEA is also produced naturally by our body in situations of stress, pain or inflammation. However, its synthesis can sometimes be disrupted by certain chronic conditions and it should also be noted that its production decreases with age.
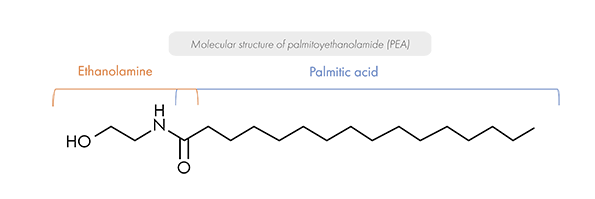
PEA is a long-chain saturated fatty acid amide belonging to the N-acylethanolamine family. It results from the combination of a palmitic acid with an ethanolamine group.
Within the family of N-acylethanolamines is the main representative of the endo-cannabinoids: anandamide. A group of chemicals that bind to specific receptors of the same name, cannabinoids play a key role in pain management1.
However, although PEA is a member of the N-acylethanolamines, it has no psychotropic properties.
Instead, PEA acts through multiple signalling pathways and different mechanisms of action in the body. It has a number of effects, such as analgesic and anti-inflammatory, which makes it an asset of choice for treating pain and inflammation, but also for contributing to relaxation and improving sleep quality.
Indeed, a meta-analysis carried out on 1,484 patients shows that taking PEA contributes to a significant reduction in chronic pain (whatever its origin: nociceptive, neuropathic and no disciplinary)2.
Furthermore, an in vivo study has shown that PEA has the ability to cross the blood-brain barrier, thus ensuring an action in the brain3.
As PEA has a long carbon chain (hydrophobic) due to its fatty acid part, this molecule has almost zero solubility in water, which explains its low bioavailability and its lesser effectiveness. This is why THERASCIENCE Laboratory uses a registered PEA Levagen®+ which guarantees its high quality and bioavailability. Levagen®+ is a palmitoylethanolamide (PEA) powder benefiting from the advanced LipiSperse® water dispersion technology. This technology allows PEA to be easily dispersed in the stomach, thereby increasing its bioavailability and absorption by the body.
To demonstrate this bioavailability, a double-blind study was conducted over 24 hours with 28 patients, half of whom were supplemented with 300 mg of Levagen®+ PEA and half with standard PEA. Patients who received Levagen®+ PEA showed a 1.75-fold increase in plasma PEA concentration compared to patients supplemented with standard PEA. This increase in absorption and bioavailability leads to a higher active concentration of PEA, thus achieving comparable efficacy to standard PEA at lower doses.
Levagen®+ PEA is a safe and well-tolerated PEA because it is an endogenous active naturally produced by the body and has the advantage of not causing any side effects4.

(1) Système cannabinoïde et douleur : quelle place en thérapeutique ? Julie Desroches, Pierre Beaulieu. Rev Med Suisse 2008; volume 4. 1505-1513
(2) Paladini, Antonella, Mariella Fusco, Teresa Cenacchi, Carlo Schievano, Alba Piroli, et Giustino Varrassi. « Palmitoylethanolamide, a Special Food for Medical Purposes, in the Treatment of Chronic Pain: A Pooled Data Meta-Analysis ». Pain Physician 19, no 2 (février 2016): 11 24.
(3) Artamonov, M., O. Zhukov, I. Shuba, L. Storozhuk, T. Khmel, V. Klimashevsky, A. Mikosha, et N. Gula. « Incorporation of Labelled N-Acylethanolamine (NAE) into Rat Brain Regions in vivo and Adaptive Properties of Saturated NAE under x-Ray Irradiation ». Ukrains’kyi Biokhimichnyi Zhurnal (1999 ) 77, no 6 (2005): 51 62.
(4) Artukoglu, Bekir Berker, Chad Beyer, Adi Zuloff-Shani, Ephraim Brener, et Michael Howard Bloch. « Efficacy of Palmitoylethanolamide for Pain: A Meta-Analysis ». Pain Physician 20, no 5 (juillet 2017): 353 62.










