
Ginfort® : Patented ginger extract highly concentrated in gingeroids, with proven effectiveness on gastrointestinal disorders
Ginfort® is a patented ginger extract, highly concentrated in polyphenolic gingeroids, containing 26% gingerols and shogaols. This extract marks a major breakthrough in the use of ginger, a plant traditionally used in Asia since 400 B.C. for its culinary and medicinal benefits, particularly in supporting digestion and soothing gastrointestinal discomfort.
Unlike traditional ginger preparations, Ginfort® delivers 12 times more gingeroids and 6-gingerol thanks to its solvent-free extraction method, and offers proven stability through its unique technology.
Carminative and prokinetic effects of Ginfort®
Ginfort® acts primarily through its gingeroids, which possess carminative and prokinetic properties. They help reduce gas, bloating, and flatulence, while promoting synchronized peristaltic movement. This process helps correct motility disorders in the stomach and small intestine—often responsible for functional dyspepsia.
Its efficacy is further enhanced by:
-
Standardization of gingeroids via HPLC: Ginfort® is standardized to contain more than 26% stable gingeroids, ensuring precise dosing;
-
Supercritical CO₂ extraction: A clean, solvent-free process that avoids undesirable contaminants;
-
Aqueosome® technology: This enhances solubility and enables prolonged release of lipophilic gingeroids (which are typically low in standard ginger extracts), thereby improving absorption of the active compounds.
This makes it possible to significantly reduce the amount of ginger required—from 2 to 4 grams to just 200 mg of Ginfort® ginger extract standardized to 26% gingeroids.

Clinical studies and safety
The efficacy of Ginfort® was demonstrated in a 28-day randomized, double-blind, placebo-controlled clinical study conducted on 50 individuals with functional dyspepsia. By administering 200 mg of Ginfort® twice daily, researchers observed a significant improvement in symptoms—postprandial fullness, abdominal bloating, and early satiety—as early as Day 14, with even more pronounced results by Day 28.
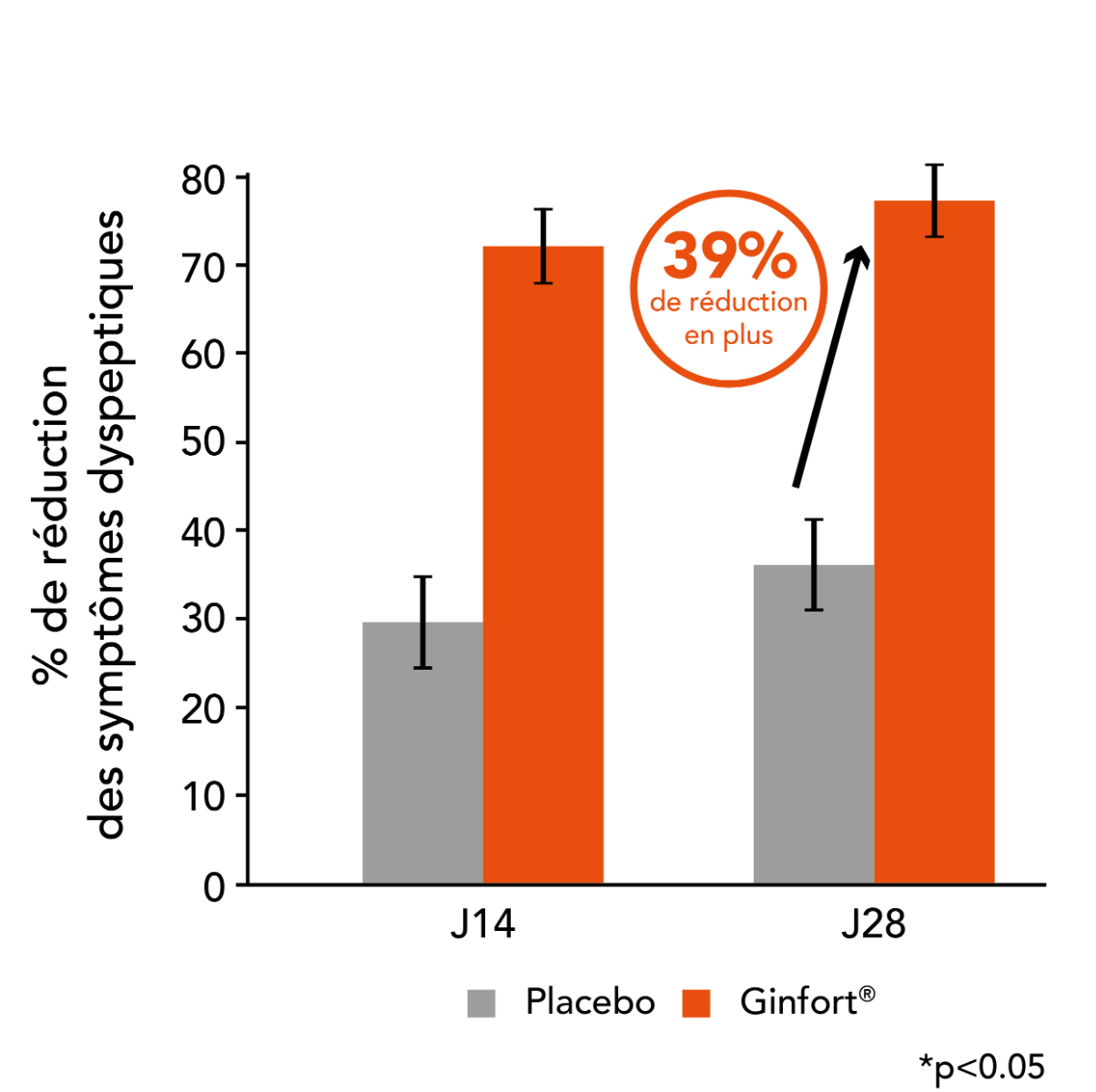
The study results showed:
-
Overall treatment efficacy: 79% of participants supplemented with Ginfort® reported a marked reduction in dyspeptic symptoms (p<0.05), compared to 21% in the placebo group — demonstrating a 58% higher efficacy for Ginfort®.
-
Symptom reduction: A 71% reduction in dyspeptic symptoms was observed in the Ginfort® group (p<0.05) versus 28% in the placebo group by Day 14; and 77% vs 38% respectively by Day 28 — showing a 39% superior efficacy for Ginfort®.
-
Safety: Ginfort® was shown to be safe, with no adverse effects reported on safety parameters.
These data clearly demonstrate that the patented Ginfort® extract stands out for its highly concentrated formula, enhanced solubility, and proven efficacy.

(1) Panda MPharm SK, Nirvanashetty PhD S, Parachur BTech VA, Krishnamoorthy MPharm C, Dey MSc S. A Rando mized, Double-Blind, Placebo Controlled, Parallel-Group, Comparative Clinical Study to Evaluate the Efficacy and Safety of OLNP-06 versus Placebo in Subjects with Functional Dyspepsia. J Diet Suppl. 2022;19(2):226-237.
(2) https://lupinepublishers.com/complementary-alternative-medicinejournal/pdf/OAJCAM.MS.ID.000203.pdf
Ginfort® is a registered trademark of Olene Life Sciences..










