
ENOTPROST® : DEPOSITED EXTRACT OF STANDARDISED FIREWEED WITH CLINICALLY PROVEN EFFICACY IN REDUCING THE SYMPTOMS OF BENIGN PROSTATIC HYPERPLASIA
Fireweed (Epilobium angustifolium) is a plant traditionally used to treat symptoms associated with benign prostatic hyperplasia (BPH). Fireweed exerts antioxidant, anti-inflammatory and antiproliferative properties thanks to its main biologically active components corresponding to flavonoids (myricetol derivatives), ellagitannins (ellagic acid and oenothein B) and phytosterols (β-sitosterol).
ENOTProst® is a fireweed extract standardised to 15% oenothein B. This active ingredient contributes to fireweed's anti-proliferative action. On the one hand, it limits the production of dihydrotestosterone (DHT) by inhibiting 5α-reductase and, on the other, reduces the aromatisation of testosterone into oestrogen, hormones involved in stimulating prostate growth.
The efficacy of ENOTProst® has been clinically demonstrated in reducing nocturia, post-micturition residual volume and improving quality of life in BPH.
A randomised, double-blind, placebo-controlled clinical trial of 128 patients supplemented daily with 500 mg ENOTProst® for 6 months showed a significant 21.7% increase in the number of people who no longer needed to get up at night to urinate, compared with a 10.2% decrease in the placebo group1.
In addition, the number of patients getting up three or more times a night was zero at the end of the study1. ENOTProst® therefore helps to improve sleep quality. Supplementation with ENOTProst® is also associated with a significant increase in the number of patients with a low post-micturition residual volume (PMRV) and a reduction in the number of patients with a PMRV greater than 100 mL1.
Improvement in quality of life was also assessed using the International Prostate Symptom Score (IPSS) questionnaire, which measures the severity of BPH symptoms. Supplementation with 500 mg ENOTProst® showed an improvement in quality of life compared with the start of the study, with a significant reduction in the IPSS score of 15% (-2 points; p<0.01), while this score increased significantly in the placebo group after 6 months (p<0.01).
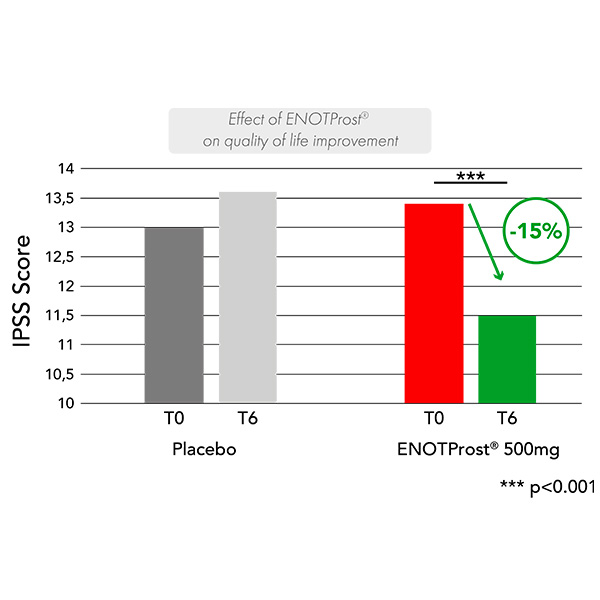

(1) Esposito, Cristina et al. “Epilobium angustifolium L. extract with high content in oenothein B on benign prostatic hyperplasia: A monocentric, randomized, double-blind, placebo-controlled clinical trial.” Biomedicine & pharmacotherapy vol. 138 (2021)
ENOTProst® is a registered trademark of EPO S.r.l.










